Breaking it Down: The Clinical Research Laboratory
Now
that we have a good understanding of how our clinic team functions to best help
our patients fight cancer, it is time to bridge the gap into clinical research.
One critical part in understanding a patient’s cancer is DNA (there are other
parts including RNA, but we will focus on DNA). After taking patient samples in
the clinic, our clinical research laboratory, often called CLIA, can extract
DNA in order to assess it. With careful handling, fancy machines, and a lot of
paperwork, the Roychowdhury Lab is better able to understand each patient’s
disease and perform experiments for the future of cancer medicine.
Our
DNA is what makes each of us unique. Each person has about 25,000 genes, or
unique segments of DNA that make everything from our hair color to our toes.
With each gene containing thousands of DNA bases (abbreviated A, C, T, or G),
humans have approximately 3 billion pieces of DNA in every cell. Because
cells often divide (we replace about 40,000 skin cells every minute!), our DNA
needs to be passed to new cells. Sometimes, this process isn’t perfect. Our
genes can fold on themselves or misplace a piece of DNA. When that happens, we
have mutations. Many mutations are harmless, but some cause problems. When a
mutation in a cell causes it to divide and grow beyond normal limits, it has
the potential to be cause cancer.
Now, in our clinic, we’ve found it very helpful to identify what these mutations are and use that information to fight a patient’s cancer. Part of effective cancer diagnosis requires a tumor biopsy, where a small number of cells from a tumor are extracted with a needle. With just these small clump of cells, our CLIA team is able to use specialized sequencing instruments to analyze every gene, every single individual DNA base—the order of the A’s, C’s, T’s, and G’s of each patient is found—that might commonly result in cancer. As little as a single base mutation (say, from what’s normally an A to a C) can cause cells to grow beyond normal control and form tumors. Looking across millions of letters for just one alteration certainly resembles the needle-in-a-haystack adage.
Now, in our clinic, we’ve found it very helpful to identify what these mutations are and use that information to fight a patient’s cancer. Part of effective cancer diagnosis requires a tumor biopsy, where a small number of cells from a tumor are extracted with a needle. With just these small clump of cells, our CLIA team is able to use specialized sequencing instruments to analyze every gene, every single individual DNA base—the order of the A’s, C’s, T’s, and G’s of each patient is found—that might commonly result in cancer. As little as a single base mutation (say, from what’s normally an A to a C) can cause cells to grow beyond normal control and form tumors. Looking across millions of letters for just one alteration certainly resembles the needle-in-a-haystack adage.
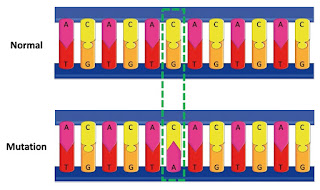 |
| Example of a DNA Mutation |
This
patient sequencing data is critical to both patient treatment and cancer
research. There are many known gene alterations (or DNA mutations) that can
cause cancer. Targeted therapy treatments have been developed for specific gene
alterations in the hope of killing any cells that possess these weapons of
disease. The sequencing data from our clinical research lab is also shared with
online platforms across the country. That way, data of hundreds to thousands of
patients can be compiled to find the most common gene abnormalities. These
genes of interest go on to be studied in many labs (including our own!) for
possible ways to combat the disease. Altogether, the biopsies done in clinic
provide the clinical research team with the tools to empower both current patient
treatment and future studies towards a world where we don’t fear cancer.



Comments
Post a Comment